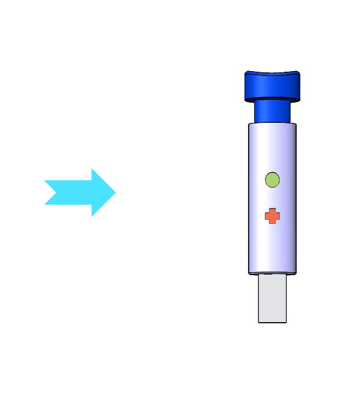
Medicortex is working towards the incorporation of proprietary brain injury biomarkers into a quick and accurate diagnostic kit that can be used by all healthcare professionals and consumers with ease. The ideal kit will not only diagnose the presence of brain injury, but will also quantify in the future its severity and indicate the precise treatment needed. In addition, the kit could become a key component of efficacy testing in all future clinical trials in TBI, and it will be used by the first responders.

Preclinical studies
Medicortex has performed preclinical research. The results showed some unique biomarkers released as biodegradation products after a head injury.

Clinical studies
Medicortex has completed three clinical studies in collaboration with three Finnish hospitals. The studies have evaluated, for example, the presence of the novel biomarker in mild TBI, specificity to TBI and its time-related appearance after the injury, and validation in child patients.
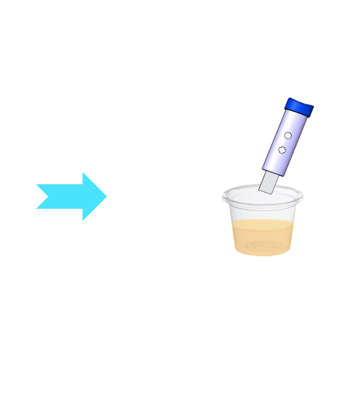

Diagnostic test prototype
The development project is on-going.
As diagnostic tests have a relatively short approval process, Medicortex believes that a prototype diagnostic kit can be presented in two years. To further expedite the entry to market, Medicortex will outsource the approval process for the CE-mark acquisition.